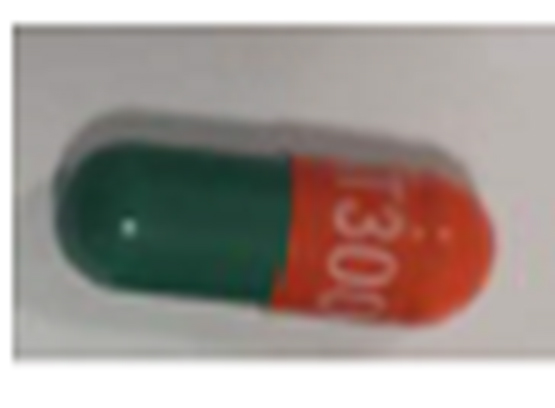
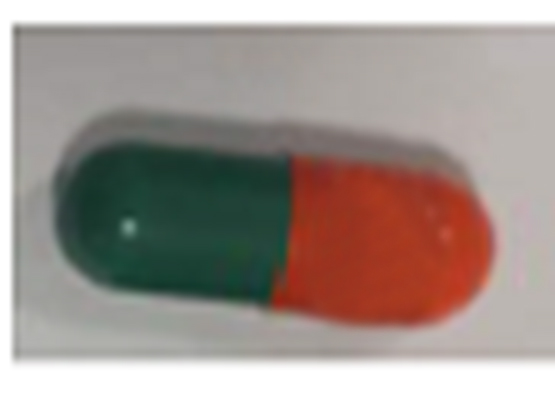
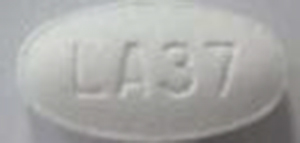| 42385-940-90 |
Atorvastatin Calcium Tablets, USP |
10 mg |
90 |
Antilipidemic drug |
Lipitor® |
|
|
|
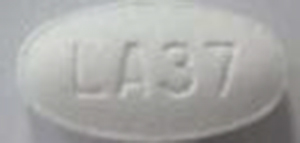
|

|
| 42385-940-01 |
Atorvastatin Calcium Tablets, USP |
10 mg |
100 |
Antilipidemic drug |
Lipitor® |
|
|
|
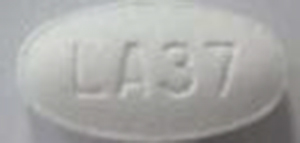
|

|
| 42385-940-05 |
Atorvastatin Calcium Tablets, USP |
10 mg |
500 |
Antilipidemic drug |
Lipitor® |
|
|
|
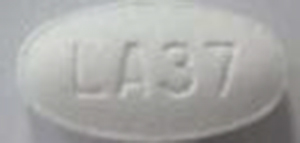
|

|
| 42385-940-11 |
Atorvastatin Calcium Tablets, USP |
10 mg |
1000 |
Antilipidemic drug |
Lipitor® |
|
|
|
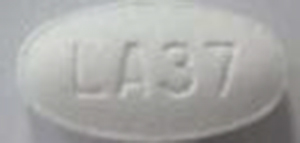
|

|
| 42385-941-90 |
Atorvastatin Calcium Tablets, USP |
20 mg |
90 |
Antilipidemic drug |
Lipitor® |
|
|
|

|

|
| 42385-941-01 |
Atorvastatin Calcium Tablets, USP |
20 mg |
100 |
Antilipidemic drug |
Lipitor® |
|
|
|

|

|
| 42385-941-05 |
Atorvastatin Calcium Tablets, USP |
20 mg |
500 |
Antilipidemic drug |
Lipitor® |
|
|
|

|

|
| 42385-941-11 |
Atorvastatin Calcium Tablets, USP |
20 mg |
1000 |
Antilipidemic drug |
Lipitor® |
|
|
|

|

|
| 42385-942-90 |
Atorvastatin Calcium Tablets, USP |
40 mg |
90 |
Antilipidemic drug |
Lipitor® |
|
|
|

|

|
| 42385-942-01 |
Atorvastatin Calcium Tablets, USP |
40 mg |
100 |
Antilipidemic drug |
Lipitor® |
|
|
|

|

|
| 42385-942-05 |
Atorvastatin Calcium Tablets, USP |
40 mg |
500 |
Antilipidemic drug |
Lipitor® |
|
|
|

|

|
| 42385-942-11 |
Atorvastatin Calcium Tablets, USP |
40 mg |
1000 |
Antilipidemic drug |
Lipitor® |
|
|
|

|

|
| 42385-943-90 |
Atorvastatin Calcium Tablets, USP |
80 mg |
90 |
Antilipidemic drug |
Lipitor® |
|
|
|

|

|
| 42385-943-01 |
Atorvastatin Calcium Tablets, USP |
80 mg |
100 |
Antilipidemic drug |
Lipitor® |
|
|
|

|

|
| 42385-943-05 |
Atorvastatin Calcium Tablets, USP |
80 mg |
500 |
Antilipidemic drug |
Lipitor® |
|
|
|

|

|
| 42385-943-11 |
Atorvastatin Calcium Tablets, USP |
80 mg |
1000 |
Antilipidemic drug |
Lipitor® |
|
|
|

|

|
| 42385-915-30 |
Efavirenz, Emtricitabine and Tenofovir Disoproxil Fumarate Tablets |
600 mg/200 mg/300 mg
|
30 |
Antiretroviral |
Atripla® |
|
- |
|

|

|
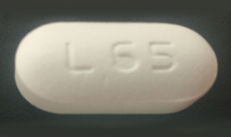
| 42385-928-30 |
Efavirenz, Lamivudine and Tenofovir Disoproxil Fumarate Tablets |
600 mg/300 mg/300 mg |
30 |
Antiretroviral |
Symfi® |
|
- |
|
|

|
| 42385-929-31 |
Efavirenz, Lamivudine and Tenofovir Disoproxil Fumarate Tablets |
400 mg/300 mg/300 mg |
30 |
Antiretroviral |
Symfi LO® |
|
- |
|

|

|
| 42385-953-30 |
Emtricitabine and Tenofovir Disoproxil Fumarate Tablets |
200 mg/300 mg |
30 |
Antiretroviral |
Truvada® |
|
|
|

|

|
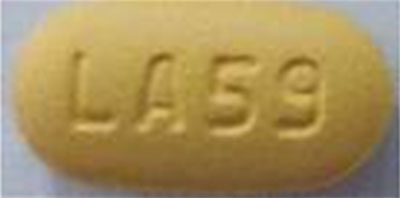
| 42385-933-60 |
Lopinavir and Ritonavir Tablets, USP |
100 mg/25 mg |
60 |
Antiretroviral |
Kaletra® |
|
- |
|
|

|
| 42385-934-12 |
Lopinavir and Ritonavir Tablets, USP |
200 mg/50 mg |
120 |
Antiretroviral |
Kaletra® |
|
- |
|

|

|
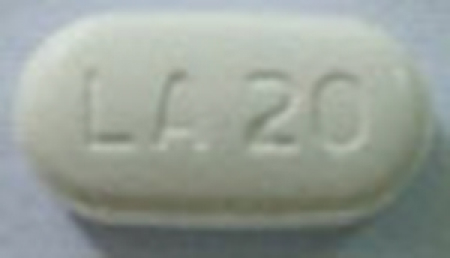
| 42385-977-90 |
Metformin Hydrochloride Extended-Release Tablets, USP |
500 mg |
90 |
Anti-diabetic |
Glucophage® XR |
|
|
|
|

|
| 42385-977-01 |
Metformin Hydrochloride Extended-Release Tablets, USP |
500 mg |
100 |
Anti-diabetic |
Glucophage® XR |
|
|
|

|

|
| 42385-977-05 |
Metformin Hydrochloride Extended-Release Tablets, USP |
500 mg |
500 |
Anti-diabetic |
Glucophage® XR |
|
|
|

|

|
| 42385-977-11 |
Metformin Hydrochloride Extended-Release Tablets, USP |
500 mg |
1000 |
Anti-diabetic |
Glucophage® XR |
|
|
|

|

|
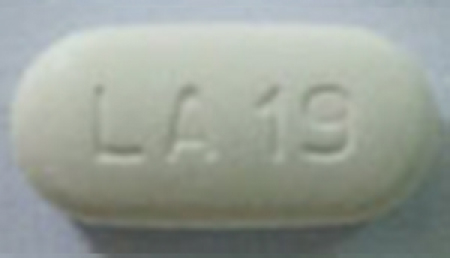
| 42385-978-01 |
Metformin Hydrochloride Extended-Release Tablets, USP |
750 mg |
100 |
Anti-diabetic |
Glucophage® XR |
|
|
|
|

|
| 42385-947-01 |
Metformin Hydrochloride Tablets, USP |
500 mg |
100 |
Anti-diabetic |
Glucophage® |
|
|
|

|

|
| 42385-947-05 |
Metformin Hydrochloride Tablets, USP |
500 mg |
500 |
Anti-diabetic |
Glucophage® |
|
|
|

|

|
| 42385-947-11 |
Metformin Hydrochloride Tablets, USP |
500 mg |
1000 |
Anti-diabetic |
Glucophage® |
|
|
|

|

|
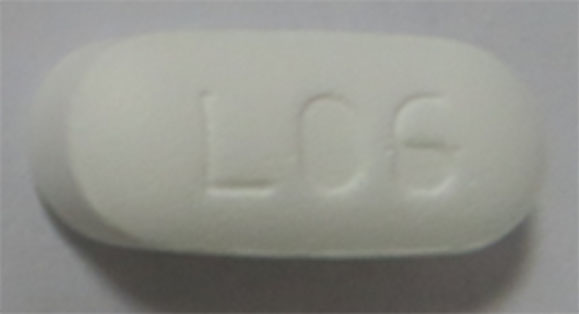
| 42385-948-01 |
Metformin Hydrochloride Tablets, USP |
850 mg |
100 |
Anti-diabetic |
Glucophage® |
|
|
|
|

|
| 42385-948-05 |
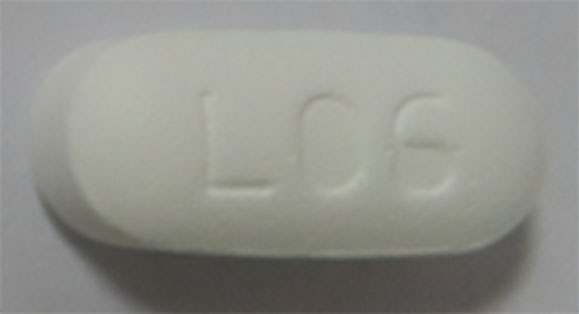
Metformin Hydrochloride Tablets, USP |
850 mg |
500 |
Anti-diabetic |
Glucophage® |
|
|
|
|

|
| 42385-948-11 |
Metformin Hydrochloride Tablets, USP |
850 mg |
1000 |
Anti-diabetic |
Glucophage® |
|
|
|

|

|
| 42385-949-01 |
Metformin Hydrochloride Tablets, USP |
1000 mg |
100 |
Anti-diabetic |
Glucophage® |
|
|
|

|

|
| 42385-949-05 |
Metformin Hydrochloride Tablets, USP |
1000 mg |
500 |
Anti-diabetic |
Glucophage® |
|
|
|

|

|
| 42385-949-11 |
Metformin Hydrochloride Tablets, USP |
1000 mg |
1000 |
Anti-diabetic |
Glucophage® |
|
|
|

|

|
| 42385-923-29 |
Pirfenidone Capsules, USP
|
267 mg |
270 |
Pyridones |
Esbriet® |
|
- |
|

|

|
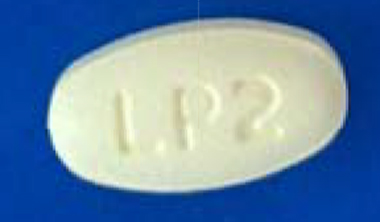
| 42385-924-99 |
Pirfenidone Tablets, USP
|
267 mg |
270 (3x90) |
Pyridones |
Esbriet® |
|
- |
|
|

|
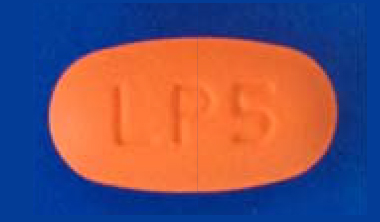
| 42385-925-90 |
Pirfenidone Tablets, USP
| 534 mg |
90 |
Pyridones |
Esbriet® |
|
- |
|
|

|
| 42385-926-90 |
Pirfenidone Tablets, USP
|
801 mg |
90 |
Pyridones |
Esbriet® |
|
- |
|

|

|
| 42385-930-60 |
Sacubitril and Valsartan Tablets |
24 mg/26 mg |
60 |
ARNI |
Entresto® |
|
|
|

|

|
| 42385-930-18 |
Sacubitril and Valsartan Tablets |
24 mg/26 mg |
180 |
ARNI |
Entresto® |
|
|
|

|

|
| 42385-931-60 |
Sacubitril and Valsartan Tablets |
49 mg/51 mg |
60 |
ARNI |
Entresto® |
|
|
|

|

|
| 42385-931-18 |
Sacubitril and Valsartan Tablets |
49 mg/51 mg |
180 |
ARNI |
Entresto® |
|
|
|

|

|
| 42385-932-60 |
Sacubitril and Valsartan Tablets |
97 mg/103 mg |
60 |
ARNI |
Entresto® |
|
|
|

|

|
| 42385-932-18 |
Sacubitril and Valsartan Tablets |
97 mg/103 mg |
180 |
ARNI |
Entresto® |
|
|
|

|

|
| 42385-946-01 |
Ursodiol Capsules, USP |
300 Mg |
100 |
PBC |
Actigall® |
|
- |
|

|

|